Unraveling the Genome May Not Cure Diseases
▶ Detecting genetic risks may have little clinical relevance.
The era of personal genomic medicine may have to wait. The genetic analysis of common disease is turning out to be a lot more complex than expected.
Since the human genome was decoded in 2003, researchers have been developing a powerful method for comparing the genomes of patients and healthy people, with the hope of pinpointing the DNA changes responsible for common diseases.
This method, called a genomewide association study, has proved technically successful despite many skeptics’initial doubts. But it has been disappointing in that the kind of genetic variation it detects has turned out to explain surprisingly little of the genetic links to most diseases.
The unexpected impasse also affects companies that offer personal genomic information and that had assumed they could inform customers of their genetic risk for diseases, based on researchers’ discoveries.
“With only a few exceptions, what the genomics companies are doing right now is recreational genomics,”said Dr.David B.Goldstein, a Duke University geneticist who was among the contributors to a recent issue of The New England Journal of Medicine that appears to be the first public attempt by scientists to make sense of this puzzling result.“The information has little or in many cases no clinical relevance,”Dr.Goldstein said.
These companies are probably not performing any useful service at present, he added.
One issue of debate among researchers is whether, despite the prospect of diminishing returns, to continue with the genomewide studies, which cost many millions of dollars apiece, or switch to a new approach like decoding the entire genomes of individual patients.
Unlike the rare diseases caused by a change affecting only one gene, common diseases like cancer and diabetes are caused by a set of several genetic variations in each person. Since these common diseases generally strike later in life, after people have had children, the theory has been that natural selection is powerless to weed them out.
The problem addressed in The New England Journal of Medicine is that these diseases were expected to be promoted by genetic variations that are common in the population. More than 100 genomewide association studies, often involving thousands of patients in several countries, have now been completed for many diseases, and some common variants have been found. But in almost all cases they carry only a modest risk for the disease. Most of the genetic link to disease remains unexplained.
Dr.Goldstein argues that the genetic burden of common diseases must be mostly carried by large numbers of rare variants. In this theory, schizophrenia, say, would be caused by combinations of 1,000 rare genetic variants, not of 10 common genetic variants.
This would be bleak news for those who argue that the common variants detected so far, even if they explain only a small percentage of the risk, will nonetheless identify the biological pathways through which a disease emerges. They argue that this would lead to the drugs that may correct the errant pathways. But if hundreds of rare variants are involved in a disease, they may implicate too much of the body’s biochemistry to be useful.
“In pointing at everything,”Dr.Goldstein writes in the journal,“genetics would point at nothing.”
Two other geneticists, Peter Kraft and David J.Hunter of the Harvard School of Public Health, also writing in the journal, largely agree with Dr.Goldstein in concluding that probably many genetic variants, rather than few,“are responsible for the majority of the inherited risk of each common disease.”
But they disagree with his belief that there will be diminishing returns from more genomewide association studies.
“There will be more common variants to find,”Dr.Hunter said.“It would be unfortunate if we gave up now.”
Dr.Goldstein, however, said it was“beyond the grasp of the genome wide association studies”to find rare variants with small effects, even by recruiting enormous numbers of patients.
“If you ask what is the fastest way for us to make progress in genetics that is clinically helpful,”he said,“I am absolutely certain it is to marshal our resources to interrogate full genomes, not in fine-tuning our analyses of common variations.”
He advocates decoding the full DNA of carefully selected patients.
Dr.Kraft and Dr.Hunter say that a person’s genetic risk of common diseases can be estimated only roughly at present but that estimates will improve as more variants are found. But that means any risk estimate offered by personal genomics companies today is unstable, Dr.Kraft said, and subject to upward or downward revision in the future.
스마터리빙
more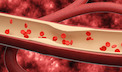 [ 건강]
[ 건강]이제 혈관 건강도 챙기자!
[현대해운]우리 눈에 보이지 않기 때문에 혈관 건강을 챙기는 것은 결코 쉽지 않은데요. 여러분은 혈관 건강을 유지하기 위해 어떤 노력을 하시나요?
 [ 건강]
[ 건강]내 몸이 건강해지는 과일궁합
 [ 라이프]
[ 라이프]벌레야 물럿거라! 천연 해충제 만들기
 [ 건강]
[ 건강]혈압 낮추는데 좋은 식품
[현대해운]혈관 건강은 주로 노화가 진행되면서 지켜야 할 문제라고 인식되어 왔습니다. 최근 생활 패턴과 식생활의 변화로 혈관의 노화 진행이 빨라지고
사람·사람들
more
[‘파친코’ 이민진 작가,인터뷰] “이민자와 취약계층 보호해야”
재미 한인 작가 이민진(57)씨가 새해 1월1일 뉴욕시장으로 취임하는 조란 맘다니(34) 뉴욕시장 당선인에 대해 “맘다니 시장이 긍정적인 변화…

김응화무용단, LA 카운티 연말축제서 ‘화관무’
김응화무용단이 지난 24일 열린 LA 카운티 연말 문화행사 제66회‘할러데이 축제’ 무대에 초청돼 한국 전통무용 ‘화관무’를 선보였다고 밝혔다…
이민단속·산불 영향 LA카운티 인구 감소
LA 카운티 인구가 올해 상당수 감소한 것으로 나타났다. 캘리포니아 주 재무국이 최근 발표한 자료에 따르면 올해 7월1일 기준 LA카운티의 인…
한인 2세, 드라마 ‘런’ 주연 맡아
권호열 세계무술총연맹 총재의 아들 에릭 권씨가 주연하는 드라마 ‘런(RUN)’ 시사회가 지난주 버지니아 애쉬번 소재 리걸 폭스 극장에서 열렸다…
[송년 행사] 코윈 퍼시픽 LA
한민족 여성네트워크(KOWIN) 퍼시픽 LA(회장 조미순)는 23일 LA 용수산에서 2025년 송년회를 열었다. 이날 행사에는 가정폭력 피해 …
많이 본 기사
- 김병기, 비위의혹에 원내대표직 전격사퇴… “국민 눈높이 못미쳐”
- “CIA가 베네수엘라 첫 지상 타격…군은 정보제공”
- 트럼프, ‘이란 예방타격’ 이스라엘 주장 두둔…중동 긴장 커지나
- 네타냐후, 트럼프에 노벨평화상 추천 이어 최고훈장까지
- 트럼프, 연말 글로벌종전외교 마무리…돌파구없었지만 동력유지
- 영주권자도 생체정보 전면 확인 1
- ICE 단속 전략 전환… ‘현장 체포’ 급증
- 트럼프·젤렌스키 회담 하루 만에… ‘… 2
- ‘韓월드컵 경기’ 멕시코 사포판 도심서 100여발 총격… “2명 사망”
- 이혜훈 “내란, 민주주의 파괴 불법행… 3
- 이 세금도입되면 부자들 떠나나?...워싱턴주지사 찬성 입장 ‘백만장자 소득세’ 논쟁 격화돼
- 젤렌스키 “美, 15년간 안전보장 제안…최대 50년 원해”
- ‘87세’ 김영옥, 하반신 마비된 손자 간병.. “누구든 아픔 있어” 고백
- “유부남과 엘리베이터서 진한 키스” 트로트 女가수 상간 ‘충격’
- [새해 달라지는 교통법규] 과속·음주운전 처벌 가주서 대폭 강화
- 401(k) 백만장자 50만명 돌파… 시간·복리 투자 ‘결실’
- 진양혜母 치매, 손범수父 입원.. “병원서 자는날 늘어남” 먹먹
- 장거리 여행 중에도 입시 준비… ‘오디오북·교육앱·팟캐스트’로
- 트럼프 “파월 해임하고 싶다…차기 의장 후보 내달 발표”
- 주택용 전기요금 내년에도 인상 전망… “중간선거에 변수”
- 새해에도 ‘부자와 서민’ 두개의 미국 뚜렷...전문가들 2026년 미국 경기전망 “부자는 소비, 서민은 할부”
- 2025년의 최대 패배자(Loser … 2
- ‘진통제 투혼’ 김민재 잊었나, ‘배은망덕’ 불타는 독일 현지 여론 “가장 실망한 선수 5위”
- “뉴욕주 ‘그린라이트 법’위헌 아니다“
- ‘은퇴 번복→폭탄선언’ 호날두 “1000골까지 계속 뛸 것”
- 워싱턴 일자리 4만개 없어졌다
- 시혹스 운명의 시즌 막판 경기...1월 3일 오후 5시 샌프란시스코서 ‘승자 독식’ 대결
- “미·중 AI경쟁 현재 점수 24대 18”…H200 수출, 전환점 되나
- ‘뉴저지 헬기 충돌’ 조종사 둘 모두 사망… “친구 사이”
- 워싱턴 일원‘슈퍼 독감’비상
- 지메일 주소변경 가능 구글, 앞부분 새 설정
- [2025 유통 결산 ①온·오프커머스] 쿠팡에 발등 찍혔지만 온라인 강세… 대형마트는 ‘고전’
- 소상공인들 “새해 비용부담 걱정 태산”...시애틀시 최저임금 새해부터 21.30달러로 인상
- 시애틀 새해맞이 이렇게 즐긴다...31일 밤 스페이스 니들 불꽃ㆍ드론 쇼 펼쳐져
- “트럼프-머스크 화해 중재자는 차기 대권 유력주자 밴스”
- 어도어 “다니엘, 뉴진스 함께 하기 어렵다..전속계약해지 통보”
- 신년 연휴에 또 비 이번주 ‘강풍주의보’
- 이민자 트럭 운전사들 “면허박탈 위법… 2
- 메타, ‘제2의 딥시크’ 마누스 인수…AI 에이전트 진용 구비
- 러 “합의 근접했다는 트럼프에 동의…우크라, 돈바스 철군해야”
- 한예슬, ♥남편과 스키장서 보낸 연말..신혼 같은 로맨틱 부부
- ESPN “애틀랜타, 김하성 영입으로 가장 큰 약점 메웠다”
- 미국이 K-푸드 주력시장… 중국 제치고 1위 부상
- 고금리·고가격·관세 ‘브레이크’… 신차 시장 썰렁
- 소프트뱅크, AI인프라 투자사 디지털브리지 40억 달러에 인수
- 故 최진실 딸 최준희, ‘개콘’서 성형 중독 고백.. “외모 만족 안 돼 “
- 트럼프, 베네수엘라 마약시설 타격 시사…첫 육상 공격 가능성
- 사유리, 아들과 기모노 가족사진.. “아빠 없어 불쌍? 웃어넘겨”
- CJ, 미국서 전통주 문화 확산 나서
- 최대 신년축제 로즈퍼레이드 D-3… 꽃차 장식 한창
1/5지식톡

-
 미 육군 사관학교 West Poin…
0
미 육군 사관학교 West Poin…
0https://youtu.be/SxD8cEhNV6Q연락처:wpkapca@gmail.comJohn Choi: 714-716-6414West Point 합격증을 받으셨나요?미 육군사관학교 West Point 학부모 모…
-
 ☝️해외에서도 가능한 한국어 선생님…
0
☝️해외에서도 가능한 한국어 선생님…
0이 영상 하나면 충분합니다!♥️상담신청문의♥️☝️ 문의 폭주로 '선착순 상담'만 진행합니다.☎️ : 02-6213-9094✨카카오톡ID : @GOODEDU77 (@골뱅이 꼭 붙여주셔야합니다…
-
 테슬라 자동차 시트커버 장착
0
테슬라 자동차 시트커버 장착
0테슬라 시트커버, 사놓고 아직 못 씌우셨죠?장착이 생각보다 쉽지 않습니다.20년 경력 전문가에게 맡기세요 — 깔끔하고 딱 맞게 장착해드립니다!장착비용:앞좌석: $40뒷좌석: $60앞·뒷좌석 …
-
 식당용 부탄가스
0
식당용 부탄가스
0식당용 부탄가스 홀세일 합니다 로스앤젤레스 다운타운 픽업 가능 안녕 하세요?강아지 & 고양이 모든 애완동물 / 반려동물 식품 & 모든 애완동물/반려동물 관련 제품들 전문적으로 홀세일/취급하는 회사 입니다 100% …
-
 ACSL 국제 컴퓨터 과학 대회, …
0
ACSL 국제 컴퓨터 과학 대회, …
0웹사이트 : www.eduspot.co.kr 카카오톡 상담하기 : https://pf.kakao.com/_BEQWxb블로그 : https://blog.naver.com/eduspotmain안녕하세요, 에듀스팟입니다…
케이타운 1번가
오피니언
 옥세철 논설위원
옥세철 논설위원2025년의 최대 패배자(Loser of The Year 2025)는?

세밑의 단상(斷想)
 메건 매카들 워싱턴포스트 칼럼니스트
메건 매카들 워싱턴포스트 칼럼니스트 [메건 매카들 칼럼] 역차별 당하는 젊은 백인 남성들
 조형숙 시인·수필가 미주문협 총무이사
조형숙 시인·수필가 미주문협 총무이사 겨울 모서리
 한영일 / 서울경제 논설위원
한영일 / 서울경제 논설위원 [만화경] 봉황의 청와대 귀환

새해 더 중요해지는 노동법 준수

연말연시, 안전하고 차분하게
 캐슬린 파커 워싱턴포스트 칼럼니스트
캐슬린 파커 워싱턴포스트 칼럼니스트 [캐슬린 파커 칼럼] 지미 라이의 마지막 희망
 유경재 나성북부교회 담임목사
유경재 나성북부교회 담임목사 [한국춘추] 미국의 힘
1/3지사별 뉴스

“뉴욕주 ‘그린라이트 법’위헌 아니다“
연방법원이 뉴욕주의 이민 신분에 관계없이 운전면허 취득을 허용하는 ‘그린라이트 법’ 시행을 막으려는 도널드 트럼프 행정부의 법적 시도를 기각시…
맨하탄 교통혼잡세 판결 내년으로 넘어가

워싱턴 일자리 4만개 없어졌다
올해 초 트럼프 대통령 취임과 함께 시작된 정부효율부(DOGE)의 대대적인 연방공무원 감원 칼바람에 올 한해동안 버지니아와 메릴랜드, DC 등…
워싱턴 일원‘슈퍼 독감’비상

트럼프, 베네수엘라 마약시설 타격 시사…첫 육상 공격 가능성
도널드 트럼프 대통령이 베네수엘라의 지상 목표물을 대상으로 한 공격이 단행됐을 가능성을 처음으로 시사했다.28일 뉴욕타임스(NYT)에 따르면 …
[새해부터 이렇게 달라진다] 최저임금 또 오르고… 유급 병가는 더 확대

오늘 하루 이 창 열지 않음 닫기 

















































.png)


댓글 안에 당신의 성숙함도 담아 주세요.
'오늘의 한마디'는 기사에 대하여 자신의 생각을 말하고 남의 생각을 들으며 서로 다양한 의견을 나누는 공간입니다. 그러나 간혹 불건전한 내용을 올리시는 분들이 계셔서 건전한 인터넷문화 정착을 위해 아래와 같은 운영원칙을 적용합니다.
자체 모니터링을 통해 아래에 해당하는 내용이 포함된 댓글이 발견되면 예고없이 삭제 조치를 하겠습니다.
불건전한 댓글을 올리거나, 이름에 비속어 및 상대방의 불쾌감을 주는 단어를 사용, 유명인 또는 특정 일반인을 사칭하는 경우 이용에 대한 차단 제재를 받을 수 있습니다. 차단될 경우, 일주일간 댓글을 달수 없게 됩니다.
명예훼손, 개인정보 유출, 욕설 등 법률에 위반되는 댓글은 관계 법령에 의거 민형사상 처벌을 받을 수 있으니 이용에 주의를 부탁드립니다.
Close
x